Introduction
In recent years, the medical field has seen a shift away
from the term concussion, redefining this injury as a mild
traumatic brain injury, or mTBI (Quatman-Yates et al.,
2020). When thought of as a TBI, the injury carries more
weight with even more serious consequences. According to the
University of Pittsburgh Medical Center (UPMC), between
1.7-3 million sports and recreation-related mTBIs are
reported annually (n.d.). While sport related mTBIs are the
most commonly researched, they can also occur from a wider
range of mechanisms including falls, motor vehicle crashes,
blast exposures, and assaults (Mayo Clinic Concussion,
2020). Additionally, the Center for Disease Control and
Prevention (CDC) reports that there are 2.53 million
emergency department visits related to TBI, with 812,000
occurring in children, showing the high incidence of brain
related injuries (2019). Due to the elevated occurrence of
mTBI’s, it is crucial that physical therapists (PTs) and
other healthcare professionals understand what a concussion
is and how to best treat it. While this article will try to
provide guidance through summarization and a general
overview, reading the clinical practice guideline (CPG) will
provide more detailed explanations and answers.
What is a Concussion/mTBI?
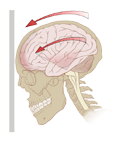
According to CPG released by the Journal of Orthopaedic &
Sports Physical Therapy in 2020, a concussion (mTBI) is
defined as “traumatic injury. That affects the brain,
induced by biomechanical forces transmitted to the head by a
blow to, or forces exerted on, the body, but that does not
result in an extended period of unconsciousness, amnesia, or
other significant neurological signs indicative of a more
severe brain injury” (Quatman-Yates et al.). Due to the
mechanism, damage can occur both to the brain and other
structures in close proximity to the brain, including the
cervicothoracic spine and vestibular system (Quatman-Yates
et al., 2020). Common symptoms of a concussion range from a
variety of cognitive, physical, emotional, and sleep
dysfunctions (Collins, 2019). Some of the most common
include headache, feeling “slowed down”, inability to
concentrate, dizziness, fogginess, fatigue, blurred/double
vision, photophobia, memory dysfunction, and balance
deficits (Collins, 2019). Due to the wide range of symptoms,
mechanisms, and structures involved, it is impossible to
define a gold standard for treatment.
Potential Pathophysiology
Despite the differences in presentation, however, there has
been some speculation around the pathophysiology of a mTBI
that would draw similarities among affected individuals. A
popular pathophysiology map was created by Giza & Hovda in
2014 and includes a step by step breakdown of a
neurometabolic pathway. This neurometabolic cascade begins
when axons are damaged or stretched following
linear/rotational acceleration/deceleration forces resulting
from the mechanism (Collins, 2019). After the acute injury,
increased depolarization of the synapses occurs, a greater
frequency of action potentials (Giza & Hovda, 2014). These
action potentials cause an increase in glutamate release
from the neurotransmitters, allowing potassium to efflux
outside of the cell (Giza & Hovda, 2014). In order to
balance the sodium-potassium ratio within the cell, the
sodium-potassium pumps use excessive ATP and work to restore
balance of potassium in the cell, resulting hyperglycolysis
(Giza & Hovda, 2014). As a byproduct of hyperglycolysis,
lactate then begins to accumulate in the cell, leading to an
increase in calcium that is sequestered into the
mitochondria to cause dysfunction (Giza & Hovda, 2014). A
positive feedback loop is then created, as the mitochondria
are less able to produce ATP and further increases the ATP
deficit caused by increased workload for the
sodium-potassium pumps (Giza & Hovda, 2014). As a result of
this process, certain enzymes within the cell are activated
to initiate apoptosis (Giza & Hovda, 2014). If this cycle is
allowed to chronically occur, the influx of calcium can lead
to compaction of the neurofilaments, microtubule
disassembly, and inflammation (Giza & Hovda, 2014). Thus,
mTBIs may lead to this chronic state of inflammation if it
is not properly managed. Despite this relatively recent
hypothesis, more research is needed to truly understand the
precise pathophysiology that occurs acutely and chronically
in those who suffer an mTBI.
CPG Foundation
The CPG released in 2020 titled Physical Therapy Evaluation
and Treatment After Concussion/Mild Traumatic Brain Injury
serves as a researched guideline for PTs to help clinically
manage mTBIs in those ages 8 and older. In general, this CPG
is not a standard of medical care and recognizes the
importance of clinical judgement and experience in patient
care. To help organize the research to date, the CPG has a
framework of 3 components: process for determining if a PT
examination is appropriate, the PT examination and
evaluation process, and development/implementation of a plan
of care. This framework is built on the principle of active
rehabilitation, defined as a 24-48 period of relative rest
post-injury followed by a phased increase in activity level
based on symptoms. When completing active rehabilitation, a
patient’s current activity level can be resumed if symptoms
are considered mild and do not cause acute exacerbation, and
a new activity level can occur if no symptoms are present at
the current level and the new level produces mild symptoms.
This recommendation is based off of the idea that symptom
provocation is necessary to guide the plan of care and
return to pre-injury functional status. When looking at mTBI
impairments that fall within the PT scope of practice, there
are 4 categories that are consistently discussed within the
CPG, including: Cervical musculoskeletal impairments,
vestibulo-oculomotor impairments, exertional tolerance
impairments, and motor function impairments. Following are
screening and diagnosis, examination, and intervention
recommendations based on these 4 impairment groups
(Quatman-Yates et al., 2020).
PT Screening and Diagnosis
While patients are often diagnosed and cleared of a serious
brain or spinal cord injury before beginning PT for mTBI,
there is strong evidence to suggest that a screening
processes should occur in PT in unique cases. This strong
evidence suggests that all individuals who have undergone a
concussive event should be screened for a concussion and
signs of severe or emergent pathology. Further, patients
without a diagnosed concussion and with a reported
concussive event should be evaluated for signs and symptoms.
Conversely, there is weak evidence for evaluating a patient
for another diagnosis in the lack of signs and symptoms of a
concussion. There is also weak evidence for the
determination of whether a PT evaluation is indicated for
those who have experienced a concussive event or have signs
and symptoms of one, however, this grade is likely due to a
lack of necessary research. A PT should always determine the
appropriateness of evaluation and treatment (Quatman-Yates
et al., 2020).
PT Examination
In a general mTBI examination, there is moderate evidence to
suggest that the examination should include elements for the
4 impairment categories recognized by the CPG and listed
above. However, there is weak evidence to suggest that the
examination should be sequenced based on patient
irritability of symptoms, due to insufficient evidence. The
lack of gold standard for sequencing is likely due to the
variety of symptoms present and patient irritability.
Regardless, the PT should consider the frequency, severity,
and ease of provocation/resolve of patient symptoms when
conducting the examination. Overall, there is no gold
standard and it is best to use clinical judgement to gather
the most information possible while also focusing on
building rapport and avoiding severe exacerbation
(Quatman-Yates et al., 2020).
Cervical musculoskeletal impairments: Weak evidence suggests
that the cervicothoracic spine should be examined if there
are any symptoms related to these areas, such as neck pain,
headache, dizziness, fatigue, difficulty focusing, and
balance deficits. While this evidence is rated as weak, the
CPG discusses that this should be performed due to the
potential of this impairment category to cause dizziness and
other non-traditional cervical/thoracic symptoms. The
examination should consist of palpation, joint mobility,
ROM, strength/endurance, and joint position error testing
(Quatman-Yates et al., 2020).
Vestibulo-oculomotor impairments: There is strong evidence
to suggest that if the patient is having signs and symptoms
consistent with BPPV (dizziness, vertigo, loss of balance,
nausea, and vomiting) the PT should perform the Dix-Hallpike
and other positional tests to rule in/out this diagnosis
(Mayo Clinic BPPV, 2020; Quatman-Yates et al., 2020).
Additionally, there is moderate evidence to suggest that if
the patient is having signs and symptoms consistent with the
vestibular or oculomotor systems, these systems should be
analyzed using tests such as ocular alignment, smooth
pursuits, saccades, vergence, visual acuity, visual motion
sensitivity, orthostatic hypotension, etc. (Quatman-Yates et
al., 2020). The vestibular-oculomotor screening tools (VOMS)
is generally used for sport to analyze these systems but may
be useful in general populations as well (Quatman-Yates et
al., 2020).
Exertional impairments: There is moderate evidence to
suggest that the PT should test a patient with a potential
concussion for orthostatic hypotension and autonomic nervous
system (ANS) dysfunction by monitoring heart rate and blood
pressure in supine, sitting, and standing. The same level of
evidence also suggests that for patients with reported
exertional deficits and desire to return to a high level of
activity, a graded exertional test based on symptoms should
be conducted on a bicycle or treadmill. However, if the
patient has cervical or vestibulo-oculomotor dysfunction,
this graded test may be performed on a bicycle according to
weak evidence. There is also weak evidence to suggest that
exertional testing may be used to determine the role of ANS
dysregulation and general fitness level, which can be
helpful to make return to play decisions. Lastly, there is
only expert recommendation to suggest that exertional
testing can be helpful in determining exertional targets for
brain healing in those that do not desire to return to
vigorous activity levels. Despite the evidence, clinical
decision making should be utilized for this category due to
the potential for exacerbation, decreased tolerance, or lack
of appropriate fitness level (Quatman-Yates et al., 2020).
Motor function impairments: According to the CPG, a moderate
level of evidence suggest that a PT should examine
static/dynamic balance, motor coordination/control, and
dual/multi-tasking. Dual and multi-tasking impairments have
growing support through evidence due to its potential
ability to determine subtle motor function impairments that
may not otherwise be observable clinically (Quatman-Yates et
al., 2020).
PT Intervention
In general for mTBI, there is strong evidence suggest that a
PT should educate their patients about potential symptoms,
impairments, and functional limitations. In doing so, the PT
should also emphasize the generally quick recovery of most
individuals with mTBI (often within 14 days). This strategy
can help reduce any rumination and negativity surrounding
the injury that could lead to delayed recovery and return to
function. Further, there is moderate evidence to suggest the
importance of self-management, relative rest within an
active rehabilitation approach, progressive re-integration
into normal activities, safety, and sleep. Moderate evidence
also suggests a personalized plan of care based on
impairments, limitations, and symptoms; however, this is
clinically common. A plan of care may begin in the first
week if appropriate, and in doing so, often leads to shorter
recovery times. Finally, if the PT feels that the patient
symptoms are not within their scope of practice (mental
health, cognition impairments, persistent migraines,
visual/auditory impairments, etc), it is important to refer
to a more appropriate healthcare professional (Quatman-Yates
et al., 2020).
Cervical musculoskeletal impairments: The CPG suggests that
there is moderate evidence to support the use of cervical
interventions to improve cervicothoracic dysfunction.
Interventions should consist of ROM, strength, postural
re-education, sensorimotor improvements, and manual therapy.
This category may also be helpful in the reduction of future
concussion risk, as imbalances within the cervical
musculature are associated with increased risk of mTBI
(Quatman-Yates et al., 2020).
Vestibulo-oculomotor impairments: After Dix-Hallpike or
other positional tests are performed and BPPV is found to be
present, there is strong evidence to suggest the use of
canalith repositioning maneuvers to decrease symptoms and
improve vestibular function. Further, moderate evidence
suggests that PTs with vestibular experience should provide
an individualized program, including a visual-motion
habituation program if vertigo is present as an impairment.
An individualized program is shown to decrease dizziness,
increase balance abilities, and lead to quicker return to
sport. However, the PT must be sure to choose interventions
wisely, as cervical dysfunction may be irritated by quick
cervical motions, such as with VOR. Finally, it is expert
opinion and ethically important to refer to another PT if
you are not familiar with or lack appropriate knowledge
within this field (Quatman-Yates et al., 2020).
Exertional impairments: The CPG suggests that there is
strong evidence for the utilization of a progressive aerobic
program guided by symptoms in patients with the desire to
return to a high level of activity. An aerobic program can
be individualized based on patient preference, goals,
functional status, and access to necessary space/equipment.
The CPG also suggests that there is no gold standard of
protocol for increasing aerobic fitness/tolerance, so time
or symptom-based protocols may be used. Moreover, there is
foundational/theoretical evidence to suggest the use of an
aerobic program for populations that do not wish to return
to a vigorous activity level to reduce deconditioning,
improve mental health, and promote brain healing. In a less
active population, aerobic activity may decrease recovery
time (Quatman-Yates et al., 2020).
Motor function impairments: There is weak evidence to
suggest the use of a motor function program for any motor
deficits that are either identified through
examination/observation or suspected, despite a growing
interest in this research body. Regardless, the use of a
motor function program may allow the patient to progress to
a higher functional level. Interventions targeting motor
function may include both static and dynamic balance, motor
coordination and control, or dual/multi-tasking activities.
Although some of these motor function impairments may be
difficult to identify clinically and may seem unimportant as
a result, they may still be beneficial for the reduction of
future mTBI risk (Quatman-Yates et al., 2020).
Limitations
While the CPG is based on the most recent evidence and is a
good tool to guide PTs through a full plan of care, there
are some limitations to adhering to these recommendations.
First off, the number of tests and measures recommended to
perform in the initial examination and re-evaluations may
not be feasible to conduct clinically within productivity
requirements and time allotments. For most of the outcome
measures listed within the CPG, the time until re-evaluation
is every 1-2 weeks, which consumes large amounts of
treatment time. Furthermore, many of the signs and symptoms
associated with each impairment category overlap, making it
more challenging to identify the accurate impairment
category. Continuing, for diagnostics, protocols, and
outcome measures, there is no recommended gold standard. PTs
must rely on clinical decision-making skills to make many of
the decisions, creating inconsistencies among practitioners.
Lastly, while there is a growing body of evidence around
mTBI, there is still limited evidence among all facets of
this topic. Much of the research conducted is also on a
young, athletic population, making it challenging to
determine what is most appropriate for a the general public
(Quatman-Yates et al., 2020).
Conclusion
Concussions, or mTBIs, have continue to push toward the
forefront of interest and conversation in the PT world. This
injury needs to be taken seriously, as it can have serious
pathophysiological, mental, and physical outcomes. In order
to best manage the condition, the CPG is an important tool
to guide PTs through the complexity of the condition.
Further, the impairment categories of cervical
musculoskeletal, vestibulo-oculomotor, exertional, and motor
function can be useful for both an examination and
interventions to allow patients the quickest and safest
return to functional activity. All in all, while there is no
one definitive way to treat mTBI, there are some general
principles to help guide decision making.
References
Center for Disease Control and Prevention. (2019). Traumatic
brain injury & concussion. Center for Disease Control and
Prevention.
https://www.cdc.gov/traumaticbraininjury/data/tbi-edhd.html
Collins, M. (2019). Rehabilitation of Prolonged Concussion
in Young Athletes [PowerPoint slides]. Children’s Hospital
of Wisconsin. PDF.
Giza, C. C., & Hovda, D. A. (2014). The new neurometabolic
cascade of concussion. Neurosurgery, 75 Suppl 4(4), S24–S33.
https://doi.org/10.1227/NEU.0000000000000505
Mayo Clinic. (2020). Benign paroxysmal positional vertigo
(BPPV). Mayo Foundation for Medical Education and Research.
https://www.mayoclinic.org/diseases-conditions/vertigo/symptoms-causes/syc-20370055
Mayo Clinic. (2020). Concusssion. Mayo Foundation for
Medical Education and Research.
https://www.mayoclinic.org/diseases-conditions/concussion/symptoms-causes/syc-20355594
Mcrory, P., Meeuwisse, W., Dvorák, J., Aubry, M., Bailes,
J., Broglio, S., Cantu, R. C., Cassidey, D., Echemendia, R.
J., Castellani, R. J., Davis, G. A., Ellenbogen, R., Emery,
C., Engebretsen, L., Feddermann-Demont, N., Giza, C. C.,
Guskiewicz, K. M., Herring, S., Iverson, G. L., Johnston, K.
M. . . (2017). Consensus statement on concussion in sport –
The 5th internaation conference on concussion in sport held
in Berlin, October 2016. British Journal of Sports Medicine,
51, 838-847. http://dx.doi.org/10.1136/bjsports-2017-097699
Quatman-Yates, C. C., Hunter-Giordano, A., Shimamura, K. K.,
Landel, R., Alsalaheen, B. A., Hanke, T. A. & McCulloch, K.
L. (2020). Physical therapy evaluation and treatment after
concussion/mild traumatic brain injury. Journal of
Orthopaedic & Sports Physical Therapy, 50(4), CPG1-CPG73.
doi: 10.2519/jospt.2020.0301
University of Pittsburgh Medical Center. (n.d.). Concussion
facts and statistics. UPMC Sports Medicine.
https://www.upmc.com/services/sports-medicine/services/concussion/facts-statistics
Last revised: December 20, 2020
by Mallory Washington, SPT